RENTACS Products
How Reverse Osmosis Works
Reverse osmosis (RO) is a filtration method that is used to remove ions and molecules from a solution by applying pressure to the solution on one side of a semipermeable or selective membrane.
Large molecules (solute) cannot cross the membrane, so they remain on one side. Water (solvent) can cross the membrane. The result is that solute molecules become more concentrated on one side of the membrane, while the opposite side becomes more dilute.
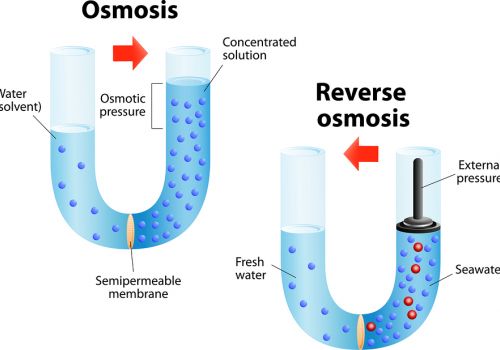
The semipermeable membrane allows the passage of water, but not ions (e.g., Na+, Ca2+, Cl-) or larger molecules (e.g., glucose, urea, bacteria).
Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration.
To illustrate, imagine a semipermeable membrane with fresh water on one side and a concentrated aqueous solution on the other side.
If normal osmosis takes place, the fresh water will cross the membrane to dilute the concentrated solution. In reverse osmosis, pressure is exerted on the side with the concentrated solution to force the water molecules through the membrane to the freshwater side.